In chemistry, hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for describing bonding properties. Hybridized orbitals are very useful in explaining of the shape of molecular orbitals for molecules, and are an integral part of valence bond theory.
The hybrids are named for the atomic orbitals involved in the hybridization. In methane (CH4) for example, a set of sp3 orbitals forms by mixing one s- and three p-orbitals on the carbon atom. The orbitals are directed toward the four hydrogen atoms, which are located at the vertices of a regular tetrahedron.
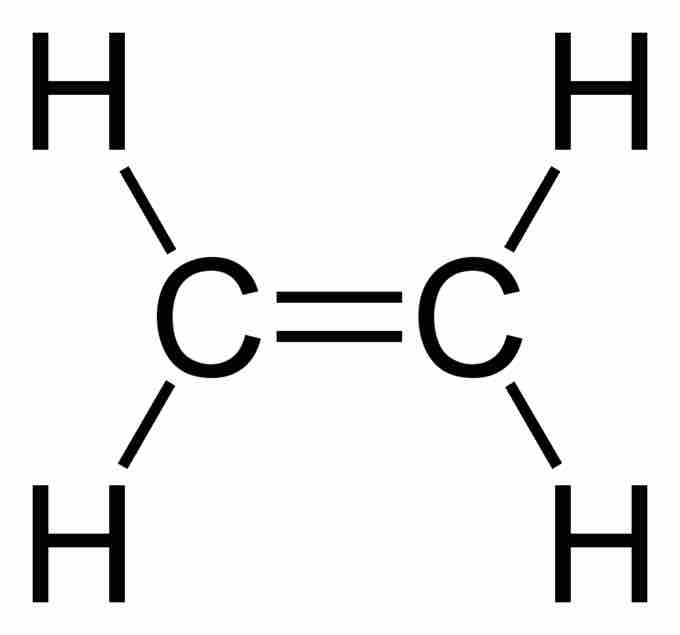
Ethene (C2H4) has a double bond between the carbons. For this molecule, carbon will sp2 hybridize. In sp2 hybridization, the 2s orbital mixes with only two of the three available 2p orbitals, forming a total of 3 sp2 orbitals with one p-orbital remaining. In ethylene (ethene), the two carbon atoms form a sigma bond by overlapping two sp2 orbitals; each carbon atom forms two covalent bonds with hydrogen by s–sp2 overlapping all with 120° angles. The pi bond between the carbon atoms forms by a 2p-2p overlap. The hydrogen-carbon bonds are all of equal strength and length, which agrees with experimental data.
Multiple bonds can also occur between dissimilar atoms. When the two O-atoms are brought up to opposite sides of the carbon atom in carbon dioxide, one of the p orbitals on each oxygen forms a pi bond with one of the carbon p-orbitals. In this case, sp hybridization leads to two double bonds.
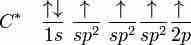
sp2 hybridization
In ethene, carbon sp2 hybridizes, because one π (pi) bond is required for the double bond between the carbons, and only three σ bonds form per carbon atom.
Ethene structure
Ethene has a double bond between the carbons.
sp hybridization explains the chemical bonding in compounds with triple bonds, such as alkynes; in this model, the 2s orbital mixes with only one of the three p-orbitals, resulting in two sp orbitals and two remaining p-orbitals. The chemical bonding in acetylene (ethyne) (C2H2) consists of sp-sp overlap between the two carbon atoms forming a sigma bond, as well as two additional pi bonds formed by p-p overlap. Each carbon also bonds to hydrogen in a sigma s-sp overlap at 180° angles.

Lewis structure of ethyne, which contains a triple bond
The sp hybridized orbitals are used to overlap with the 1s hydrogen orbitals and the other carbon atom. The remaining, non-hybridized p-orbitals overlap for the double and triple pi bonds.
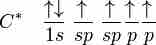
sp hybridisation
In this model, the 2s orbital mixes with only one of the three p-orbitals, resulting in two sp-orbitals and two remaining unchanged p-orbitals.