In chemistry, bond polarity is the separation of electric charge along a bond, leading to a molecule or its chemical groups having an electric dipole or dipole moment.
Electrons are not always shared equally between two bonding atoms. One atom might exert more of a force on the electron cloud than the other; this pull is called electronegativity. Electronegativity measures a particular atom's attraction for electrons. The unequal sharing of electrons within a bond leads to the formation of an electric dipole (a separation of positive and negative electric charge). Partial charges are denoted as δ+ (delta plus) and δ- (delta minus), symbols that were introduced by Christopher Ingold and his wife Hilda Usherwood in 1926.
Atoms with high electronegativity values—such as fluorine, oxygen, and nitrogen—exert a greater pull on electrons than do atoms with lower electronegativity values. In a bond, this can lead to unequal sharing of electrons between atoms, as electrons will be drawn closer to the atom with higher electronegativity.
Bonds can fall between one of two extremes, from completely nonpolar to completely polar. A completely nonpolar bond occurs when the electronegativity values are identical and therefore have a difference of zero. A completely polar bond, or ionic bond, occurs when the difference between electronegativity values is large enough that one atom actually takes an electron from the other. The terms "polar" and "nonpolar" usually refer to covalent bonds. To determine the polarity of a covalent bond using numerical means, find the difference between the electronegativity of the atoms; if the result is between 0.4 and 1.7, then, generally, the bond is polar covalent.
The hydrogen fluoride (HF) molecule is polar by virtue of polar covalent bonds; in the covalent bond, electrons are displaced toward the more electronegative fluorine atom.
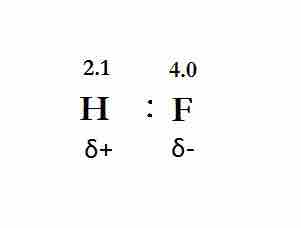
The polar covalent bond, HF.
The more electronegative (4.0 > 2.1) fluorine pulls the electrons in the bond closer to it, forming a partial negative charge. The resulting hydrogen atom carries a partial positive charge.