In heteronuclear diatomic molecules, atomic orbitals only mix when the electronegativity values are similar. In carbon monoxide (CO), the oxygen 2s orbital is much lower in energy than the carbon 2s orbital, so the degree of mixing is low. The g and u subscripts no longer apply because the molecule lacks a center of symmetry.
In hydrogen fluoride (HF), the hydrogen 1s orbital can mix with the fluorine 2pz orbital to form a sigma bond because experimentally, the energy of 1s of hydrogen is comparable with 2p of fluorine. The HF electron configuration reflects that the other electrons remain in three lone pairs and that the bond order is one.
While MOs for homonuclear diatomic molecules contain equal contributions from each interacting atomic orbital, MOs for heteronuclear diatomics contain different atomic orbital contributions. Orbital interactions that produce bonding or antibonding orbitals in heteronuclear diatomics occur if there is sufficient overlap between atomic orbitals, as determined by their symmetries and similarity in orbital energies.
Examples of Heteronuclear Diatomic Molecules
In hydrogen fluoride, HF, symmetry allows for overlap between the H 1s and F 2s orbitals, but the difference in energy between the two atomic orbitals prevents them from interacting to create a molecular orbital. Symmetry also allows for overlap between the H 1s and F 2pz orbitals, and these two atomic orbitals have a small energy separation; they therefore interact, creating σ and σ* MOs and a molecule with a bond order of one.

Hydrogen fluoride
The hydrogen fluoride molecule.
Hydrogen chloride, HCl, is a diatomic molecule consisting of a hydrogen atom H and a chlorine atom Cl connected by a covalent single bond. Since the chlorine atom is much more electronegative than the hydrogen atom, the covalent bond between the two atoms is quite polar. Consequently, the molecule has a large dipole moment with a negative partial charge δ- at the chlorine atom and a positive partial charge δ+ at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other polar solvents).
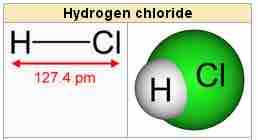
Hydrogen chloride
Hydrogen chloride is a diatomic molecule.
Carbon monoxide, CO, has a total of 10 valence electrons. To satisfy the octet rule for the carbon, the two atoms form a triple bond with six shared electrons in three bonding molecular orbitals. Since four of the shared electrons come from the oxygen atom and only two from carbon, one of the bonding orbitals is occupied by two electrons from oxygen.

Carbon monoxide
Carbon monoxide.
Chlorine monofluoride can convert metals and non-metals to their fluorides, releasing Cl2 in the process; it converts tungsten to tungsten hexafluoride and selenium to selenium tetrafluoride, for example. ClF is a colorless gas at room temperature and is stable even at high temperatures. When cooled to −100 °C, ClF condenses as a pale yellow liquid. Many of its properties are intermediate between its parent halogens, Cl2 and F2.

Chlorine monofluoride
The interhalogen molecule, chlorine monofluoride.
Heteronuclear Diatomic Molecules and Their Dipole Moments
To determine exact polarity, dipole moment (in Debye) can be calculated as the product of the separated charges (Q) and distance between them (r) in Angstroms:
Finding the value of Q can be challenging, but the value is easily converted from the percent ionic character of a bond—simply convert the percent to decimal by dividing by 100; r is simply the bond length.
Sample problem: What is the dipole moment of the Cl-F molecule with a bond length of 163 picometers (163 x 10-12 m) and an 11 percent ionic character? (1D = 3.36 x 10-30 Cm)(1e- = 1.60 x 10-19 C)
Solve for the value in Debye (this value represents the molecule with 100 percent ionic character):
For 11 percent ionic character:
D = 7.8 x .11 = .86 D