Concept
Version 11
Created by Boundless
Molecular Geometries
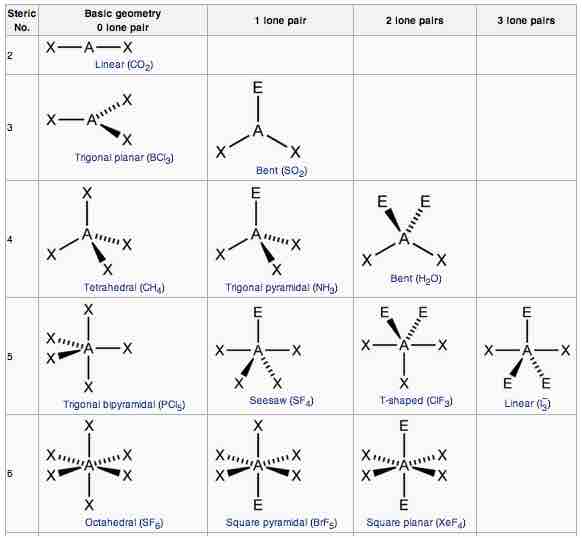
AXE method
The A represents the central atom; the X represents the number of sigma bonds between the central atoms and outside atoms; and the E represents the number of lone electron pairs surrounding the central atom. The sum of X and E, known as the steric number, is also associated with the total number of hybridized orbitals used by valence bond theory.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: