Concept
Version 13
Created by Boundless
Lone Electron Pairs
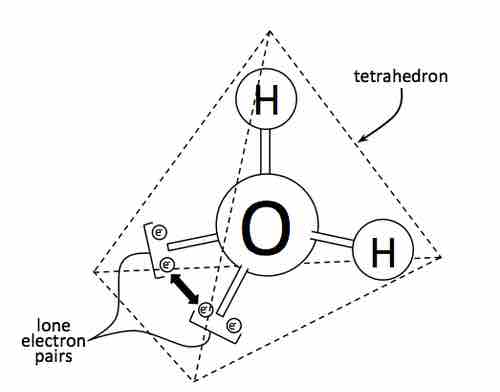
The effect of the lone pair on water
Although the oxygen atom is tetrahedrally coordinated, the bonding geometry (shape) of the H2O molecule is described as bent.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Tetrahedral_Structure_of_Water.png."
https://commons.wikimedia.org/wiki/File:Tetrahedral_Structure_of_Water.png
Wikimedia
CC0 1.0 Universal.