An oxoacid (sometimes called an oxyacid) is an acid that contains oxygen. To be more specific, an oxoacid is an acid that:
- contains oxygen
- contains at least one other element
- has at least one hydrogen atom bonded to oxygen
- forms an ion by the loss of one or more protons in solution.
Examples of oxoacids include:
- Carboxylic acids
- Sulfuric acid
- Nitric acid
- Phosphoric acid
Halogen oxoacids include hypochlorous acid (HOCl); chlorous acid(HOClO); chloric acid(HOClO2); oerchloric acid(HOClO3); oerbromic acid (HOBrO3)
All oxoacids have the acidic hydrogen bound to an oxygen atom, so bond strength (length) is not a factor, similar to binary nonmetal acids; instead, the main determining factor for an oxacid's relative strength has to do with the central atom's electronegativity (X), as well as the number of O atoms around that central atom.

Sulphuric acid
Drops of the concentrated oxoacid sulfuric acid (sulphuric acid) rapidly dehydrate a piece of cotton towel.
Electronegativity of the Central Atom
Consider the simple oxyacids HOI (hypoiodous acid), HOBr (hypobromous acid), and HOCl (hypochlorous acid). These acids can be arranged in order of their pKa values and, by extension, their relative strengths:
HOCl pKa = 7.5 < HOBr pKa = 8.6 < HOI pKa = 10.6
Recall that smaller values of pKa correspond to greater acid strength. Therefore, HOCl is the strongest acid and HOI is weakest, and acid strength decreases as the central halogen descends on the periodic table.
The strength of the acid is determined by the central atom's electronegativity relative to the surround atoms in the molecule. Because Cl is the most electronegative, it draws the bulk of the electrons in the HOCl molecule toward itself; because H and Cl are on opposite ends of the molecule, Cl pulls at the electrons in the H-O bond, thereby weakening it. The weaker the H-O bond, the more easily the H+ can ionize in water, and the stronger the acid.
Number of Oxygen Atoms Around the Central Atom
Consider the family of chlorooxoacids, which are arranged below in order of pKa values:
HOClO3 pKa = -8 < HOClO2 pKa = -1.0 < HOClO pKa = 1.92 < HOCl pKa = 7.53
The strongest acid is perchloric acid on the left, and the weakest is hypochlorous acid on the far right. Notice that the only difference between these acids is the number of oxygens bonded to chlorine. As the number of oxygens increases, so does the acid strength; again, this has to do with electronegativity. Oxygen is a highly electronegative element, and the more oxygen atoms present, the more that the molecule's electron density will be pulled off the O-H bond, weakening it and creating a stronger acid.
Carboxylic Acids
Carboxylic acids are an important subclass of organic oxoacids, characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group. A carboxyl group (or carboxy) is a functional group consisting of a carbonyl (RR'C=O) and a hydroxyl (R-O-H), which has the formula -C(=O)OH, usually written as -COOH or -CO2H.
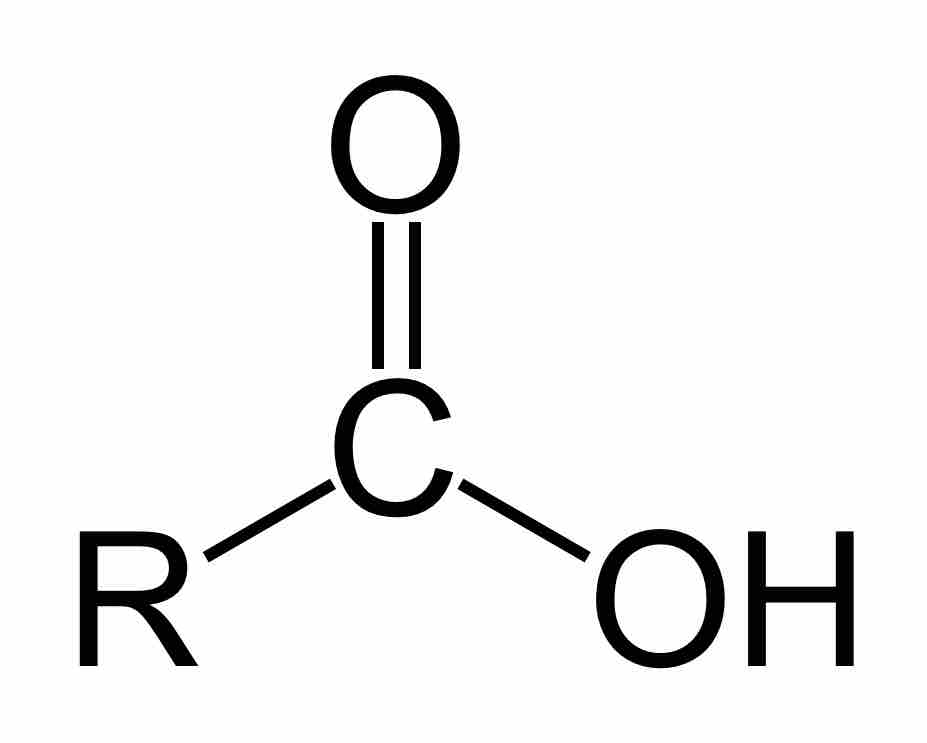
A carboxylic acid
Carboxylic acids are organic oxoacids characterized by the presence of at least one carboxyl group, which has the formula -C(=O)OH, usually written as -COOH or -CO2H.
Carboxylic acids are the most common type of organic acid. Among the simplest examples are formic acid H-COOH, which occurs in ants, and acetic acid CH3-COOH, which gives vinegar its sour taste. Acids with two or more carboxyl groups are called dicarboxylic, tricarboxylic, etc. The simplest dicarboxylic example is oxalic acid (COOH)2, which is just two connected carboxyls. Mellitic acid is an example of a hexacarboxylic acid. Other important natural examples include citric acid (in lemons) and tartaric acid (in tamarinds).
Salts and esters of carboxylic acids are called carboxylates. When a carboxyl group is deprotonated, its conjugate base, a carboxylate anion, forms. Carboxylate ions are resonance stabilized, and this increased stability makes carboxylic acids more acidic than alcohols. Carboxylic acids can be seen as reduced or alkylated forms of the Lewis acid carbon dioxide; under some circumstances they can be decarboxylated to yield carbon dioxide.