Section 3
Buffer Effectiveness
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts

Relative Amounts of Acid and Base
The pH of a buffer depends on the ratio [base]/[acid] rather than on the particular concentration of a specific solution.

Absolute Concentrations of the Acid and Conjugate Base
For an effective buffer, there must be enough acid/conjugate base to consume all newly added ions so that the pH is maintained.
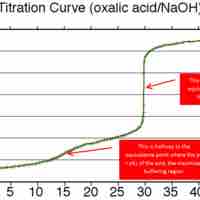
Buffer Range and Capacity
A buffer's capacity is the pH range where it works as an effective buffer, preventing large changes in pH upon addition of an acid or base.