Equilibrium Constant Expression
The law of chemical equilibrium states that, at any given temperature a chemical system reaches a state in which a particular ratio of reactant and product activities has a constant value. This constant is known as the equilibrium constant. For the generic reaction
the equilibrium constant denoted by Keq is given by
where [X] is the activity of X. The activity of X is equal to the concentration of X if it is a gas or liquid. The activity is equal to 1 if its a pure liquid or solid. In other words, pure solids and liquids do not affect the equilibrium constant as long as there is enough for the reaction to proceed. Their activity is 1, so they do not need to be written in the equilibrium constant. Since activity is a dimensionless quantity, the equilibrium constant, Keq, is also a dimensionless quantity.
By convention, the equilibrium concentrations of the substances appearing on the right hand side of the chemical equation (the products) are always placed in the numerator of the equilibrium constant expression; the concentrations of the substances appearing on the left hand side of the chemical equation (the reactants) are placed in the denominator. The larger the value of the equilibrium constant, the more the reaction proceeds to completion. Irreversible reactions can be thought to have an infinite equilibrium constant since there are no reactants left .
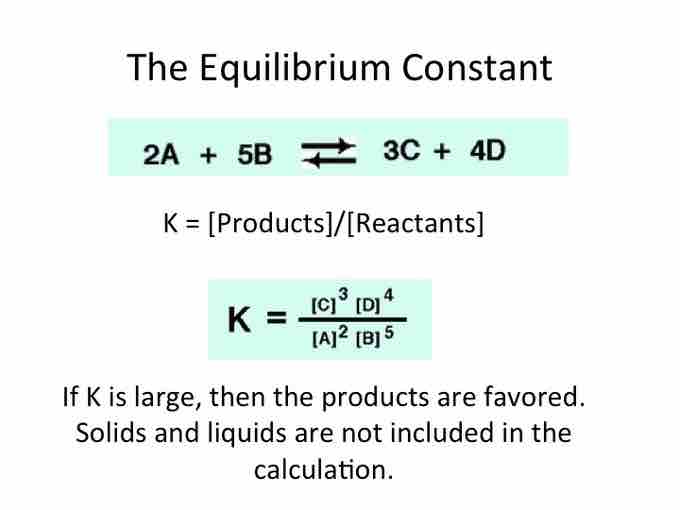
The Equilibrium Constant
The equilibrium constant,denoted by K, is the ratio of products to reactants at equilibrium.
Example:
Take the reaction:
The forward reaction is:
The reverse reaction is:
The equilibrium constant expression for this reaction is:
The progress of an equilibrium reaction can be visualized. At time = 0, the rate of the forward reaction is high and the rate of the reverse reaction is low. As the reaction proceeds, the rate of the forward reaction decreases and the rate of the reverse reaction increases, until both occur at the same rate, reaching equilibrium.