Concept
Version 14
Created by Boundless
Protein Structure
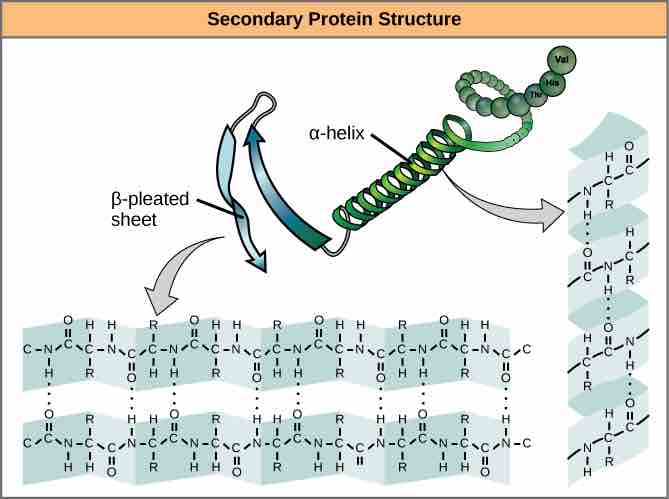
Secondary structure
The α-helix and β-pleated sheet form because of hydrogen bonding between carbonyl and amino groups in the peptide backbone. Certain amino acids have a propensity to form an α-helix, while others have a propensity to form a β-pleated sheet.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, Proteins. October 16, 2013."
http://cnx.org/content/m44402/latest/Figure_03_04_07.jpg
OpenStax CNX
CC BY 3.0.