The Electromagnetic Spectrum
The electromagnetic (EM) spectrum is the range of all possible frequencies of electromagnetic radiation . The electromagnetic spectrum extends from below the low frequencies used for modern radio communication to gamma radiation at the short-wavelength (high-frequency) end, thereby covering wavelengths of thousands of kiilometers down to those of a fraction of the size of an atom (approximately an angstrom). The limit for long wavelengths is the size of the universe itself.
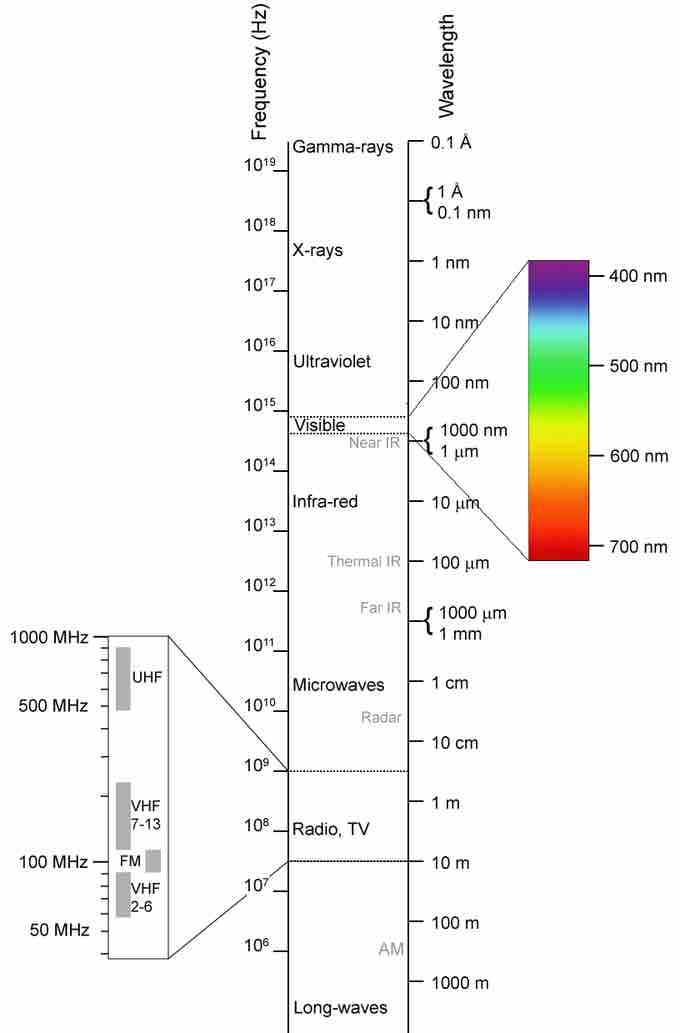
Electromagnetic spectrum
This shows the electromagnetic spectrum, including the visible region, as a function of both frequency (left) and wavelength (right).
Maxwell's equations predicted an infinite number of frequencies of electromagnetic waves, all traveling at the speed of light. This was the first indication of the existence of the entire electromagnetic spectrum. Maxwell's predicted waves included waves at very low frequencies compared to infrared, which in theory might be created by oscillating charges in an ordinary electrical circuit of a certain type. In 1886, the physicist Hertz built an apparatus to generate and detect what are now called radio waves, in an attempt to prove Maxwell's equations and detect such low-frequency electromagnetic radiation. Hertz found the waves and was able to infer (by measuring their wavelength and multiplying it by their frequency) that they traveled at the speed of light. Hertz also demonstrated that the new radiation could be both reflected and refracted by various dielectric media, in the same manner as light.
Filling in the Electromagnetic Spectrum
In 1895, Wilhelm Röntgen noticed a new type of radiation emitted during an experiment with an evacuated tube subjected to a high voltage. He called these radiations 'X-rays' and found that they were able to travel through parts of the human body but were reflected or stopped by denser matter such as bones. Before long, there were many new uses for them in the field of medicine.
The last portion of the electromagnetic spectrum was filled in with the discovery of gamma rays. In 1900, Paul Villard was studying the radioactive emissions of radium when he identified a new type of radiation that he first thought consisted of particles similar to known alpha and beta particles, but far more penetrating than either. However, in 1910, British physicist William Henry Bragg demonstrated that gamma rays are electromagnetic radiation, not particles. In 1914, Ernest Rutherford (who had named them gamma rays in 1903 when he realized that they were fundamentally different from charged alpha and beta rays) and Edward Andrade measured their wavelengths, and found that gamma rays were similar to X-rays, but with shorter wavelengths and higher frequencies.
The relationship between photon energy and the radiation's frequency and wavelength is illustrated as the following equilavent equation:
Most parts of the electromagnetic spectrum are used in science for spectroscopic and other probing interactions as ways to study and characterize matter. Also, radiation from various parts of the spectrum has many other uses in communications and manufacturing.