Tetrahedral Complexes
In tetrahedral molecular geometry, a central atom is located at the center of four substituent atoms, which form the corners of a tetrahedron. The bond angles are approximately 109.5° when all four substituents are the same. This geometry is widespread, particularly for complexes where the metal has d0 or d10 electron configuration.
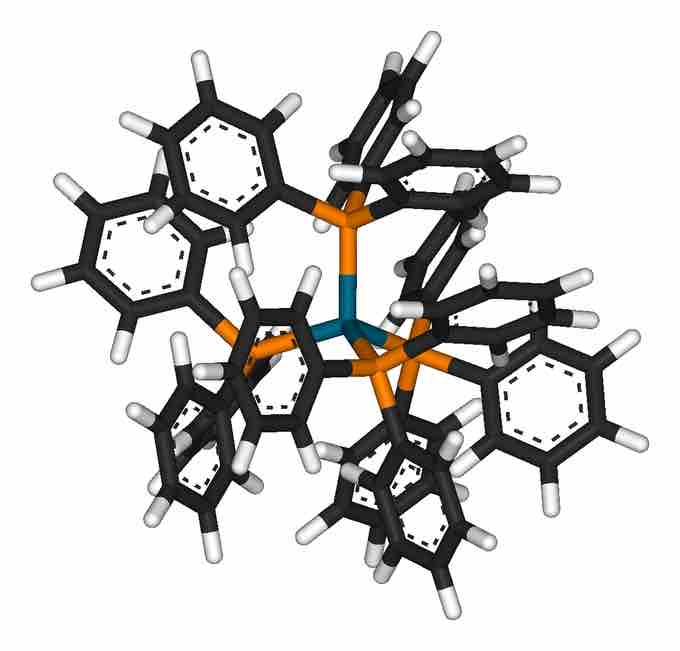
Tetrakis(triphenylphosphine)palladium
3-dimensional representation of tetrahedral Tetrakis(triphenylphosphine)palladium
For example, tetrakis(triphenylphosphine)palladium(0), a popular catalyst, and nickel carbonyl, an intermediate in nickel purification, are tetrahedral. Many complexes with incompletely filled d-subshells are tetrahedral as well—for example, the tetrahalides of iron(II), cobalt(II), and nickel(II).
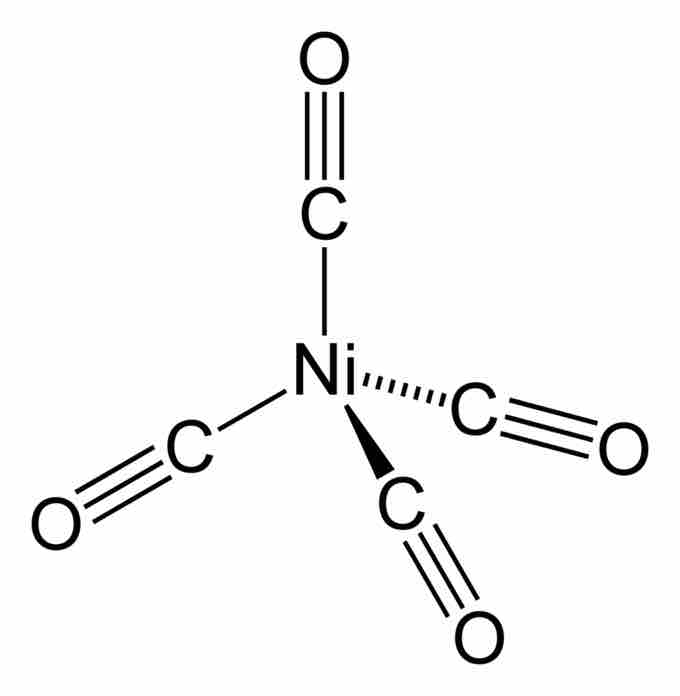
Nickel carbonyl
2-dimensional representation of tetrahedral nickel carbonyl.
Tetrahedral complexes have ligands in all of the places that an octahedral complex does not. Therefore, the crystal field splitting diagram for tetrahedral complexes is the opposite of an octahedral diagram. The dx2−dy2 and dz2 orbitals should be equally low in energy because they exist between the ligand axis, allowing them to experience little repulsion. In contrast, the dxy,dyz, and dxz axes lie directly on top of where the ligands go. This maximizes repulsion and raises energy levels.
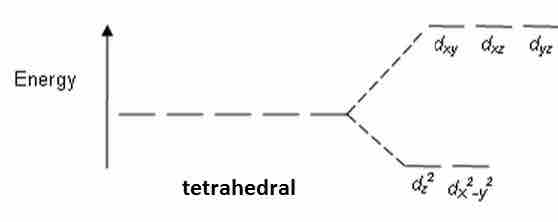
Tetrahedral CFT splitting
Notice the energy splitting in the tetrahedral arrangement is the opposite for the splitting in octahedral arrangements.
Square Planar Complexes
In square planar molecular geometry, a central atom is surrounded by constituent atoms, which form the corners of a square on the same plane. The geometry is prevalent for transition metal complexes with d8 configuration. This includes Rh(I), Ir(I), Pd(II), Pt(II), and Au(III). Notable examples include the anticancer drugs cisplatin [PtCl2(NH3)2] and carboplatin.
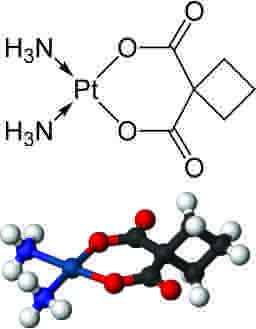
Carboplatin
2- and 3-dimensional representations of the anti-cancer drug carboplatin
In principle, square planar geometry can be achieved by flattening a tetrahedron. As such, the interconversion of tetrahedral and square planar geometries provides a pathway for the isomerization of tetrahedral compounds. For example, tetrahedral nickel(II) complexes such as NiBr2(PPh3)2 undergo this change reversibly.
The removal of a pair of ligands from the z-axis of an octahedron leaves four ligands in the x-y plane. Therefore, the crystal field splitting diagram for square planar geometry can be derived from the octahedral diagram. The removal of the two ligands stabilizes the dz2 level, leaving the dx2-y2 level as the most destabilized. Consequently, the dx2-y2 remains unoccupied in complexes of metals with the d8 configuration. These compounds typically have sixteen valence electrons (eight from ligands, eight from the metal).
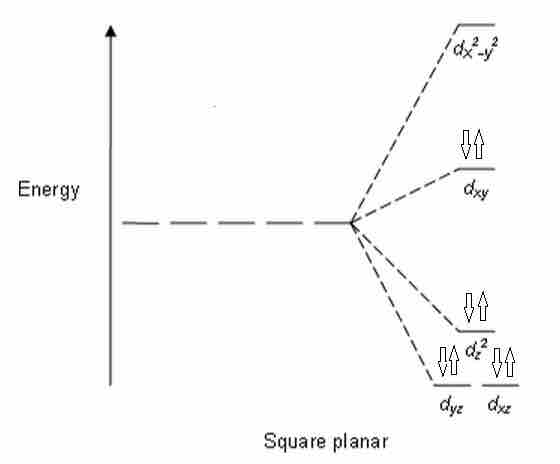
CFT energy diagram for square planar complexes
Notice how the dx2 - y2 orbital is unfilled.