Electron Configuration of Cations and Anions
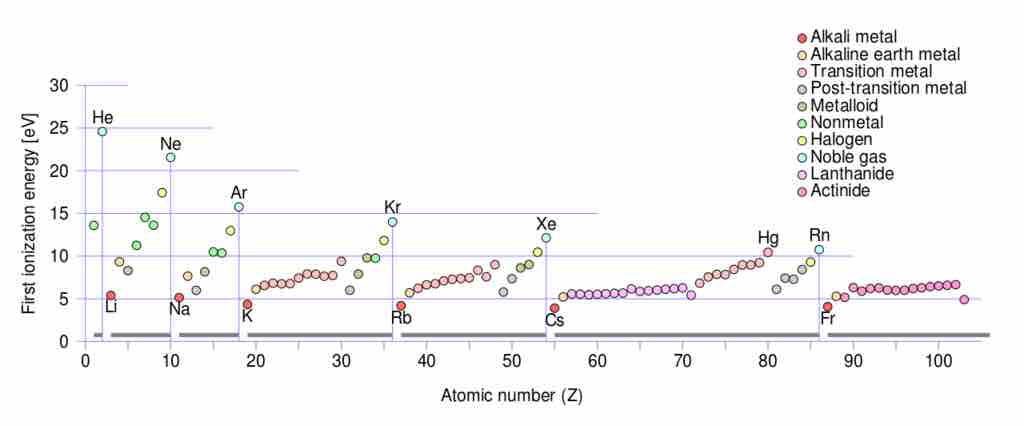
First ionization energy
Periodic trends for ionization energy (IE) vs. atomic number: note that within each of the seven periods the IE (colored circles) of an element begins at a minimum for the first column of the Periodic table (the alkali metals), and progresses to a maximum for the last column (the noble gases) which are indicated by vertical lines and labelled with a noble gas element symbol, and which also serve as lines dividing the 7 periods. Note that the maximum ionization energy for each row diminishes as one progresses from row 1 to row 7 in a given column, due to the increasing distance of the outer electron shell from the nucleus as inner shells are added.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: