Reactions of Alkenes and Alkynes
Alkenes and alkynes are generally more reactive than alkanes due to the electron density available in their pi bonds. In particular, these molecules can participate in a variety of addition reactions and can be used in polymer formation.
Addition Reactions
Unsaturated hydrocarbons can participate in a number of different addition reactions across their double or triple bonds.
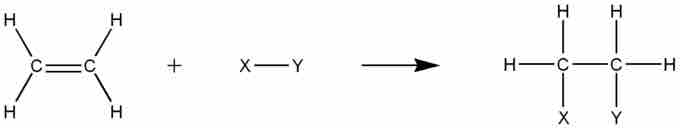
Addition reactions
Alkenes participate in a variety of addition reactions.
These addition reactions include catalytic hydrogenation (addition of H2), halogenation (reaction with X2, where X is a halogen), and hydrohalogenation (reaction with H-X, where X is a halogen), among others.
Cycloaddition
Alkenes undergo diverse cycloaddition reactions. Most notable is the Diels–Alder reaction with 1,3-dienes to give cyclohexenes.
.jpg)
Diels-Alder reaction
Here, the reaction of 1,3-butadiene (the diene) reacts with ethylene (the dienophile) to produce cyclohexene.
This general reaction has been extensively developed, and electrophilic alkenes and alkynes are especially effective dienophiles. Cycloaddition processes involving alkynes are often catalyzed by metals.
Oxidation
Oxidation of alkynes by strong oxidizing agents such as potassium permanganate or ozone will yield a pair of carboxylic acids. The general reaction can be pictured as:
By contrast, alkenes can be oxidized at low temperatures to form glycols. At higher temperatures, the glycol will further oxidize to yield a ketone and a carboxylic acid:
Here, we have 3-methyl-2-butene oxidizing to form acetone and acetic acid.
Hydrogenation
In the presence of a catalyst—typically platinum, palladium, nickel, or rhodium—hydrogen can be added across a triple or a double bond to take an alkyne to an alkene or an alkene to an alkane. In practice, it is difficult to isolate the alkene product of this reaction, though a poisoned catalyst—a catalyst with fewer available reactive sites—can be used to do so. As the hydrogen is immobilized on the surface of the catalyst, the triple or double bonds are hydrogenated in a syn fashion; that is to say, the hydrogen atoms add to the same side of the molecule.
Halogenation
Alkenes and alkynes can also be halogenated with the halogen adding across the double or triple bond, in a similar fashion to hydrogenation. The halogenation of an alkene results in a dihalogenated alkane product, while the halogenation of an alkyne can produce a tetrahalogenated alkane.
Hydrohalogenation
Alkenes and alkynes can react with hydrogen halides like HCl and HBr. Hydrohalogenation gives the corresponding vinyl halides or alkyl dihalides, depending on the number of HX equivalents added. The addition of water to alkynes is a related reaction, except the initial enol intermediate converts to the ketone or aldehyde. If the alkene is asymmetric, the reaction will follow Markovnikov's rule—the halide will be added to the carbon with more alkyl substituents.
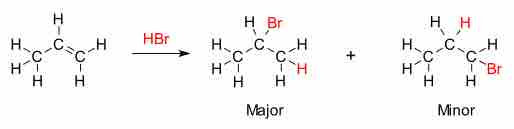
Markovnikov's rule
This rule dictates that the addition of a hydrogen halide (HX, in the case of HBr) to an alkene will lead to a product where the hydrogen is attached to the carbon with fewer alkyl substituents, while the halide group is attached to the carbon with more alkyl substituents.
Hydration
Water can be added across triple bonds in alkynes to yield aldehydes and ketones for terminal and internal alkynes, respectively. Hydration of alkenes via oxymercuration produces alcohols. This reaction takes place during the treatment of alkenes with a strong acid as the catalyst.