A metal can refer to an element, compound, or alloy that is a good conductor of both electricity and heat. Example metals include gold, sodium, copper, iron, and many other elements. Metals are usually malleable, ductile, and shiny.
Density of Metals
Metals typically consist of close-packed atoms, meaning that the atoms are arranged like closely packed spheres. In a metal, atoms readily lose electrons to form positive ions (cations). Those ions are surrounded by de-localized electrons, which are responsible for the conductivity. The solid produced is held together by electrostatic interactions between the ions and the electron cloud, which are called metallic bonds.
Metals are shiny and lustrous with a high density. They have very high melting and boiling points because metallic bonding is very strong, so the atoms are reluctant to break apart into a liquid or gas.

Sodium Metal
Sodium metal is soft enough to be cut with a plastic knife.
Conductivity of Metals
Metals in general are conductive, with high electrical conductivity and high thermal conductivity. Typically they are malleable and ductile, deforming under stress without cleaving. For example, hitting a metal with a hammer will "dent" the metal, not shatter it into pieces.
The electrical and thermal conductivities of metals originate from the fact that their outer electrons are delocalized. This means the electrons are not locked into any one atom but can move freely throughout the metal. Metals can be viewed as a collection of atoms embedded in a sea of electrons, which are highly mobile. This is very instrumental in the conductivity of the metal.
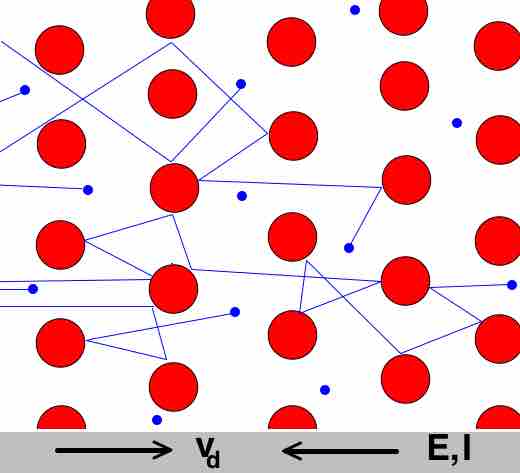
Sea of Electrons
The "sea of electrons" is free to flow about the crystal of positive metal ions.
Metals are usually inclined to form cations through electron loss. An example is the reaction with oxygen in the air to form oxides over various timescales (iron rusts over years, while potassium burns in seconds). The transition metals (such as iron, copper, zinc, and nickel) are slower to oxidize because they form a passivating layer of oxide that protects the interior. Others, like palladium, platinum, and gold, do not react with the atmosphere at all. Some metals form a barrier layer of oxide on their surface, which cannot be penetrated by further oxygen molecules. As a result, they retain their shiny appearance and good conductivity for many decades (like aluminium, magnesium, some steels, and titanium).