Elements
A chemical element is a pure substance that consists of one type of atom. Each atom has an atomic number, which represents the number of protons that are in the nucleus of a single atom of that element. The periodic table of elements is ordered by ascending atomic number.
The chemical elements are divided into the metals, the metalloids, and the non-metals. Metals, typically found on the left side of the periodic table, are:
- often conductive to electricity
- malleable
- shiny
- sometimes magnetic.
Aluminum, iron, copper, gold, mercury and lead are metals.
In contrast, non-metals, found on the right side of the periodic table (to the right of the staircase), are:
- typically not conductive
- not malleable
- dull (not shiny)
- not magnetic.
Examples of elemental non-metals include carbon and oxygen.
Metalloids have some characteristics of metals and some characteristics of non-metals. Silicon and arsenic are metalloids.
As of November, 2011, 118 elements have been identified (the most recently identified was ununseptium, in 2010). Of these 118 known elements, only the first 98 are known to occur naturally on Earth. The elements that do not occur naturally on Earth are the synthetic products of man-made nuclear reactions. 80 of the 98 naturally-occurring elements are stable; the rest are radioactive, which means they decay into lighter elements over timescales ranging from fractions of a second to billions of years.
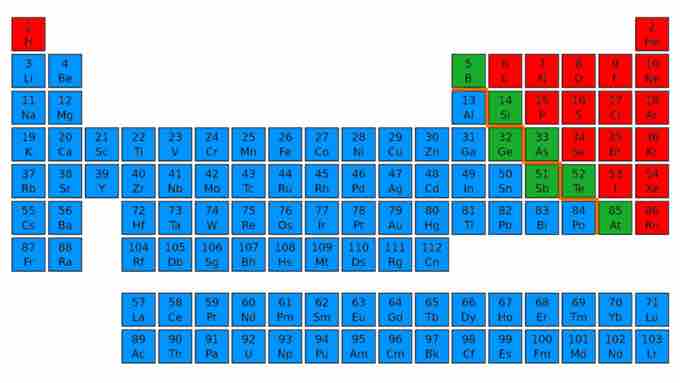
The periodic table
The periodic table shows 118 elements, including metals (blue), nonmetals (red), and metalloids (green).
Hydrogen and helium are by far the most abundant elements in the universe. However, iron is the most abundant element (by mass) in the composition of the Earth, and oxygen is the most common element in the layer that is the Earth's crust.
Although all known chemical matter is composed of these elements, chemical matter itself constitutes only about 15% of the matter in the universe. The remainder is dark matter, a mysterious substance that is not composed of chemical elements. Dark matter lacks protons, neutrons, or electrons.
Compounds
Pure samples of isolated elements are uncommon in nature. While the 98 naturally occurring elements have all been identified in mineral samples from the Earth's crust, only a small minority of them can be found as recognizable, relatively pure minerals. Among the more common of such "native elements" are copper, silver, gold, and sulfur. Carbon is also commonly found in the form of coal, graphite, and diamonds. The noble gases (e.g., neon) and noble metals (e.g., mercury) can also be found in their pure, non-bonded forms in nature. Still, most of these elements are found in mixtures.
When two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. Most elements on Earth bond with other elements to form chemical compounds, such as sodium (Na) and Chloride (Cl), which combine to form table salt (NaCl). Water is another example of a chemical compound. The two or more component elements of a compound can be separated through chemical reactions.
Chemical compounds have a unique and defined structure, which consists of a fixed ratio of atoms held together in a defined spatial arrangement by chemical bonds. Chemical compounds can be:
- molecular compounds held together by covalent bonds
- salts held together by ionic bonds
- intermetallic compounds held together by metallic bonds
- complexes held together by coordinate covalent bonds.
Pure chemical elements are not considered chemical compounds, even if they consist of diatomic or polyatomic molecules (molecules that contain only multiple atoms of a single element, such as H2 or S8).