The rate law for a chemical reaction is an equation that relates the reaction rate with the concentrations or partial pressures of the reactants. For the general reaction
In this equation, [A] and [B] express the concentrations of A and B, respectively, in units of moles per liter. The exponents x and y vary for each reaction, and they must be determined experimentally; they are not related to the stoichiometric coefficients of the chemical equation. Lastly, k is known as the rate constant of the reaction. The value of this coefficient k will vary with conditions that affect reaction rate, such as temperature, pressure, surface area, etc. A smaller rate constant indicates a slower reaction, while a larger rate constant indicates a faster reaction.
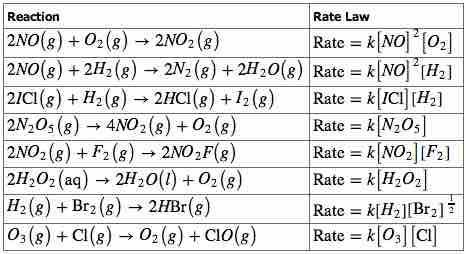
Rate laws for various reactions
A variety of reaction orders are observed. Note that the reaction order is unrelated to the stoichiometry of the reactions; it must be determined experimentally.
Reaction Order
To reiterate, the exponents x and y are not derived from the balanced chemical equation, and the rate law of a reaction must be determined experimentally. These exponents may be either integers or fractions, and the sum of these exponents is known as the overall reaction order. A reaction can also be described in terms of the order of each reactant. For example, the rate law
Example 1
A certain rate law is given as
The reaction is first-order in hydrogen, one-half-order in bromine, and
Example 2
The reaction between nitric oxide and ozone,