Concept
Version 8
Created by Boundless
The Incomplete Octet
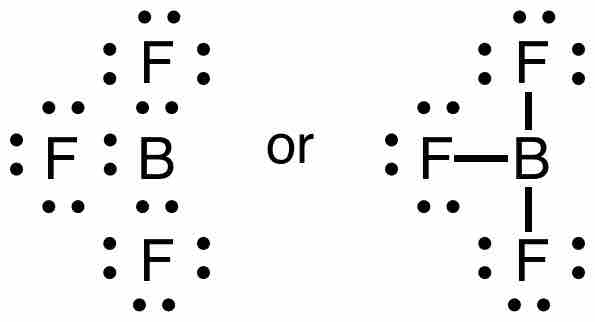
Lewis structure of boron trifluoride
Each pair of dots represents a pair of electrons. When placed between two atoms, the electrons are in a bond. A bond can be drawn as a line between two atoms, which also indicates two electrons. Notice that the central boron atom has only 6 electrons in the final Lewis diagram/structure of this molecule.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Boundless."
http://s3.amazonaws.com/figures.boundless.com/506f0850e4b0dfd51f0b0f57/BF3+and+BF3NH3.png
Amazon Web Services
CC BY-SA 3.0.