Concept
Version 15
Created by Boundless
Explanation of Valence Bond Theory
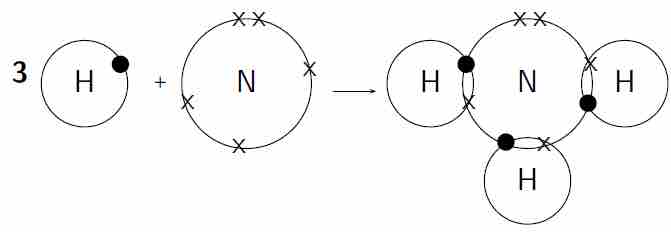
Covalent bonding in a molecule of ammonia
Each hydrogen atom needs one more electron to complete its valence energy shell. The nitrogen atom needs three more electrons to complete its valence energy shell. Therefore, three pairs of electrons must be shared between the four atoms involved. The nitrogen atom will share three of its electrons so that each of the hydrogen atoms now has a complete valence shell. Each of the hydrogen atoms will share its electron with the nitrogen atom to complete its valence shell.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Free High School Science Texts Project, Chemical Bonding - Grade 10 (11) [CAPS]. October 9, 2012."
http://cnx.org/content/m38131/latest/
OpenStax CNX
CC BY 3.0.