The Mechanism of Protein Synthesis
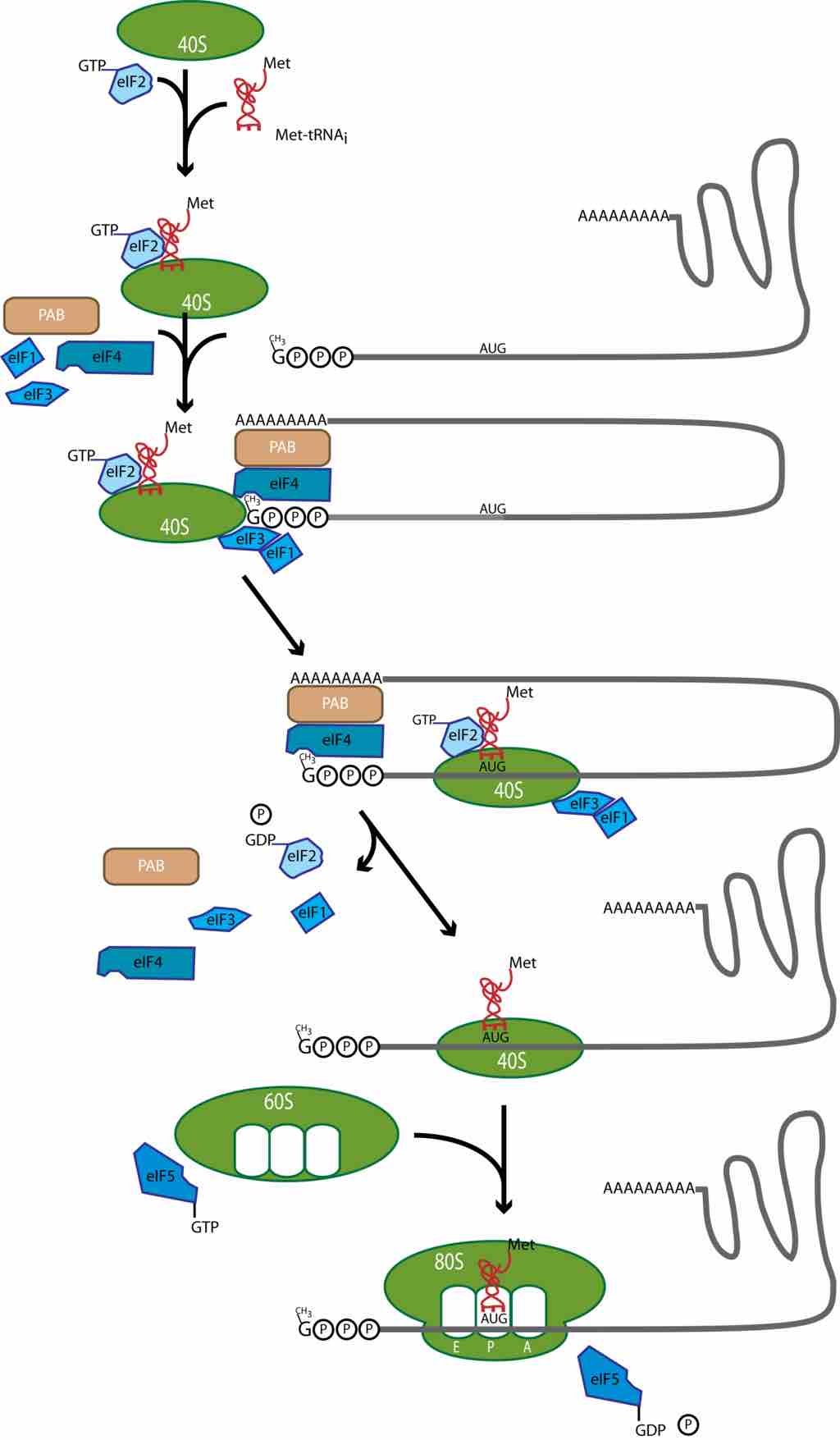
Translation initiation in eukaryotes.
In eukaryotes, a preinitiation complex forms made of the small 40S subunit, the initiator Met-tRNAi, and eIF2-GTP. This preinitiation complex binds to the 5'-m7G cap of the mRNA with the help of other eIFS and PAB, which binds the poly(A) tail of the mRNA, and loops the tail to the cap. Once at the cap, the preinitiation complex slides along the mRNA until it encounters the initiator AUG codon. There, GTP is hydrolyzed by eIF2 and the Met-tRNAi is loaded onto the AUG. Next, eIF5-GTP recruits the 60S large ribosomal subunit to the 40S subunit at the AUG and hydrolyzes GTP. This allows the large ribosomal subunit to assemble on top of the small subunit, generating the intact 80S ribosome, and places the Met-tRNAi in the P site of the intact ribosome . The ribosome A site is positioned over the second codon in the mRNA reading frame, and translation elongation can begin.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: