Concept
Version 14
Created by Boundless
Microscopic Anatomy
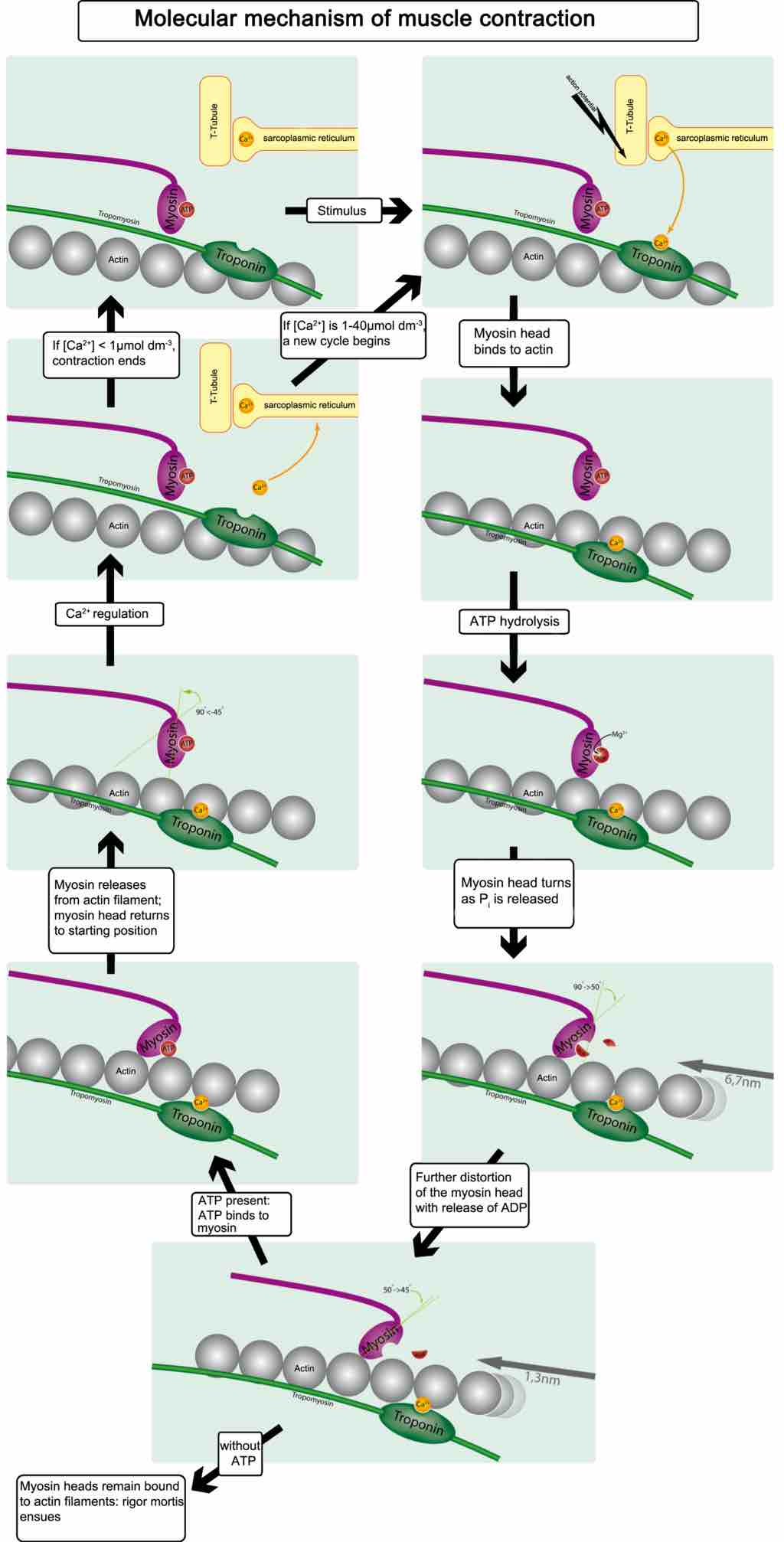
Muscle Contraction and Actin-Myosin Interactions
Skeletal muscle contracts following activation by an action potential. Binding of Acetylcholine at the motor end plate leads to intracellular calcium release and interactions between myofibrils, eliciting contraction.
This diagram illustrates the molecular mechanism of muscular contraction. With application of a stimulus, the myosin head binds to actin, resulting in ATP hydrolysis. The myosin head turns as P is released and is further distorted with the release of ATP. When ATP is present, it binds to myosin, which releases from the actin filament returning the myosin head to starting position. CA2 regulation either causes contractions to end or a new cycle to begin. When myosin heads remain bound to actin filaments, rigor mortis ensues.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Muskel-molekulartranslation."
http://en.wikipedia.org/wiki/File:Muskel-molekulartranslation.png
Wikipedia
CC BY-SA.