Calorimetry
Overview
Calorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry is performed with a calorimeter. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. The word calorimetry is derived from the Latin word calor, meaning heat. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of calorimetry.
Calorimetry requires that the material being heated have known thermal properties, i.e. specific heat capacities . The classical rule, recognized by Clausius and by Kelvin, is that the pressure exerted by the calorimetric material is fully and rapidly determined solely by its temperature and volume; this rule is for changes that do not involve phase change, such as melting of ice. There are many materials that do not comply with this rule, and for them, more complex equations are required than those below.
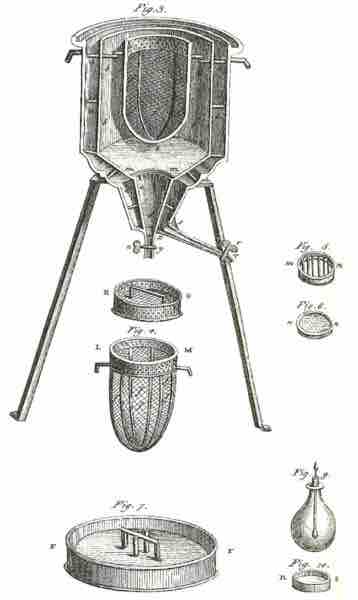
Ice Calorimeter
The world's first ice-calorimeter, used in the winter of 1782-83, by Antoine Lavoisier and Pierre-Simon Laplace, to determine the heat evolved in variouschemical changes; calculations which were based on Joseph Black's prior discovery of latent heat. These experiments mark the foundation of thermochemistry.
Basic Calorimetry at Constant Value
Constant-volume calorimetry is calorimetry performed at a constant volume. This involves the use of a constant-volume calorimeter (one type is called a Bomb calorimeter). For constant-volume calorimetry:
where δQ is the increment of heat gained by the sample, CV is the heat capacity at constant volume, cv is the specific heat at constant volume, and ΔT is the change in temperature.
Measuring Enthalpy Change
To find the enthalpy change per mass (or per mole) of a substance A in a reaction between two substances A and B, the substances are added to a calorimeter and the initial and final temperatures (before the reaction started and after it has finished) are noted. Multiplying the temperature change by the mass and specific heat capacities of the substances gives a value for the energy given off or absorbed during the reaction:
Dividing the energy change by how many grams (or moles) of A were present gives its enthalpy change of reaction. This method is used primarily in academic teaching as it describes the theory of calorimetry. It does not account for the heat loss through the container or the heat capacity of the thermometer and container itself. In addition, the object placed inside the calorimeter shows that the objects transferred their heat to the calorimeter and into the liquid, and the heat absorbed by the calorimeter and the liquid is equal to the heat given off by the metals.
Constant-Pressure Calorimetry
A constant-pressure calorimeter measures the change in enthalpy of a reaction occurring in solution during which the atmospheric pressure remains constant. An example is a coffee-cup calorimeter, which is constructed from two nested Styrofoam cups and a lid with two holes, allowing insertion of a thermometer and a stirring rod. The inner cup holds a known amount of a solute, usually water, that absorbs the heat from the reaction. When the reaction occurs, the outer cup provides insulation. Then
where Cp is the specific heat at constant pressure, ΔH is the enthalpy of the solution, ΔT is the change in temperature, W is the mass of the solute, and M is the molecular mass of the solute. The measurement of heat using a simple calorimeter, like the coffee cup calorimeter, is an example of constant-pressure calorimetry, since the pressure (atmospheric pressure) remains constant during the process. Constant-pressure calorimetry is used in determining the changes in enthalpy occurring in solution. Under these conditions the change in enthalpy equals the heat (Q=ΔH).