The Oil-Drop Experiment
The Oil-Drop Experiment, otherwise known as the Millikan Oil-Drop Experiment, is one of the most influential studies in the history of physical science.
Performed by Robert Millikan and Harvey Fletcher in 1911, the experiment was designed to determine the charge of a single electron, otherwise known as the elementary electric charge.
Millikan designed his experiment to measure the force on oil droplets between two electrodes.
He used an atomizer to spray a mist of tiny oil droplets into a chamber, which included a hole. Some droplets would fall through this hole and into a chamber, where he measured their terminal velocity and calculated their mass.
Millikan then exposed the droplets to X-rays, which ionized molecules in the air and caused electrons to attach to the oil droplets, thus making them charged. The top and bottom of the chamber were attached to a battery, and the potential difference between the top and bottom produced an electric field that acted on the charged oil drops.
Adjusting the voltage perfectly, Millikan was able to balance the force of gravity (which was exerted downward) with the force of the electric field on the charged particles (which was exerted upward), causing the oil droplets to be suspended in mid-air.
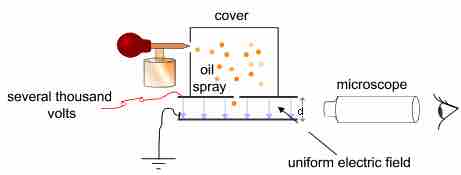
Simplified scheme of Millikan's oil-drop experiment
This apparatus has a parallel pair of horizontal metal plates. A uniform electric field is created between them. The ring has three holes for illumination and one for viewing through a microscope. Special oil for vacuum apparatus is sprayed into the chamber, where drops become electrically charged. The droplets enter the space between the plates and can be controlled by changing the voltage across the plates.
Millikan then calculated the charge on particles suspended in mid-air. His assumptions were that the force of gravity, which is the product of mass (m) and gravitational acceleration (g), was equal to the force of the electric field (the product of the charge (q) and the electric field (E)):
Since he already knew the mass of the oil droplets and the acceleration due to gravity (9.81 m/s^2), as well as the energy of the x-rays he was using, he was able to calculate the charge.
Although the charge of each droplet was unknown, Millikan adjusted the strength of the X-rays ionizing the air and measured many values of (q) from many different oil droplets. In each instance, the charge measured was a multiple of 1.5924(17)×10−19 C. Thus, it was concluded that the elementary electric charge was 1.5924(17)×10−19 C.
The results were very accurate. The calculated value from the Oil-Drop Experiment differs by less than one percent of the current accepted value of 1.602176487(40)×10−19 C.
The Oil-Drop Experiment was tremendously influential at the time, not only for determining the charge of an electron, but for helping prove the existence of particles smaller than atoms. At the time, it was not fully accepted that protons, neutrons, and electrons existed.