The concept of polarity is very broad and can be applied to molecules, light, and electric fields. For the purposes of this atom, we focus on its meaning in the context of what is known as dielectric polarization—the separation of charges in materials.
Dielectrics
A dielectric is an insulator that can be polarized by an electric field, meaning that it is a material in which charge does not flow freely, but in the presence of an electric field it can shift its charge distribution. Positive charge in a dielectric will migrate towards the applied field, while negative charges will shift away. This creates a weak local field within the material that opposes the applied field.
Different materials will react differently to an induced field, depending on their dielectric constant. This constant is the degree of their polarizability (the extent to which they become polarized).
Atomic Model
The most basic view of dielectrics involves considering their charged components: protons and electrons. If an electric field is applied to an atom, the electrons in the atom will migrate away from the applied field. The protons, however, remain relatively exposed to the field. This separation creates a dipole moment, as shown in .
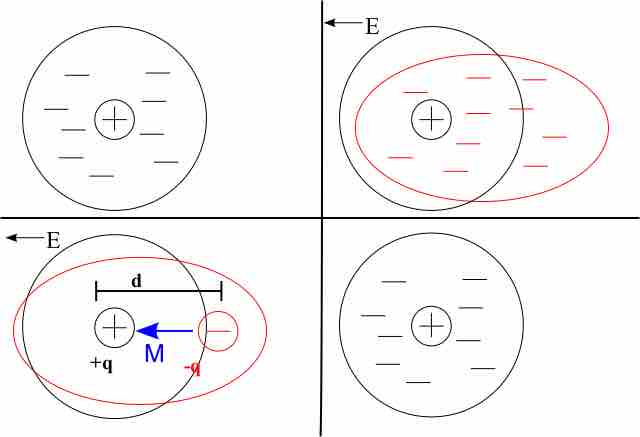
Reaction of an Atom to an Applied Electric Field
When an electric field (E) is applied, electrons drift away from the field. Their average location is displaced from the average location of the protons (which hasn't moved) by a distance of d. The atom's dipole moment is represented by M.
Dipole Polarization
On the molecular level, polarization can occur with both dipoles and ions. In polar bonds, electrons are more attracted to one nucleus than to the other. One example of a dipole molecule is water, (H2O), which has a bent shape (the H-O-H angle is 104.45°) and in which the oxygen pulls electron density away from the H atoms, leaving the H relatively positive and the O relatively negative, as shown in .
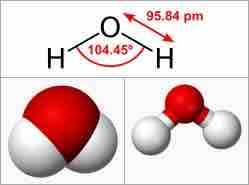
Water Molecule
Water is an example of a dipole molecule, which has a bent shape (the H-O-H angle is 104.45°) and in which the oxygen pulls electron density away from the H atoms, leaving the H relatively positive and the O relatively negative.
When a dipolar molecule is exposed to an electric field, the molecule will align itself with the field, with the positive end towards the electric field and the negative end away from it.
Ionic Polarization
Ionic compounds are those that are formed from permanently charge-separated ions. For example, table salt (NaCl) is formed from Na+ and Cl- ions that are not formally bound to one another through a chemical bond, but interact very strongly due to their opposite charges.
Ions are still free from one another and will naturally move at random. If they happen to move in a way that is asymmetrical, and results in a greater concentration of positive ions in one area and a greater concentration of negative ions in another, the sample of ionic compound will be polarized—a phenomenon is known as ionic polarization.