All living organisms contain either DNA or RNA. These molecules contain genetic information that defines (or at least influences) all of the organism's characteristics. In both DNA and RNA, electrostatics plays a major role. For the purpose of this atom we focus specifically on DNA.
DNA is a large macromolecule that (in three-dimensional space) forms the shape of a double helix, as shown in . The "backbone" of each helix is formed from alternating deoxyribose and phosphate subunits as illustrated in . Each deoxyribose is connected to a base that extends between the two backbones. Four bases can be found in DNA: adenine, guanine, cytosine and thymine. Their ordering in sequence determines the genetic characteristics of the parent organism.
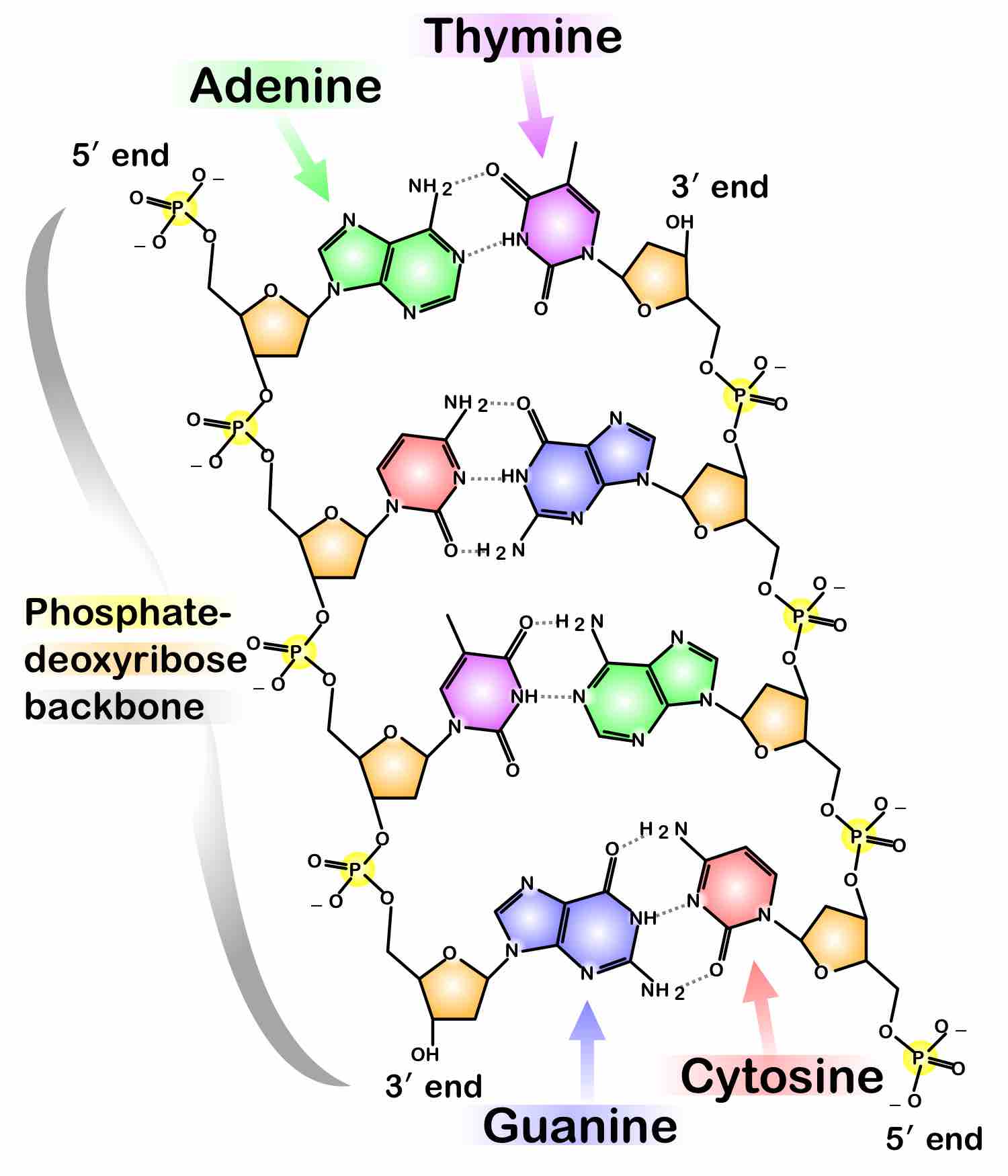
Phosphate in DNA
The complex molecules that make up our DNA are held together by a phosphate-deoxyribose backbone, as shown.
The bases of each strand of DNA are positioned close to one another but not as a result of formal chemical bonds. Electrostatic interactions, in this case otherwise known as hydrogen bonds, are what hold these bonds together.
In , (in the instance of guanine and cytosine) there are three instances in which hydrogen atoms are attracted to nearby nitrogen and oxygen atoms (denoted by dashed lines). For adenine and thymine, there are two such hydrogen bonds. These interactions can be modeled as electrostatic interactions—electrons from the oxygen and nitrogen atoms are negative, and are attracted to the exposed nuclei (which are positive) of the nearby hydrogen atoms. These hydrogen bonds are relatively weak, but their sum is strong enough to hold the two DNA strands together.
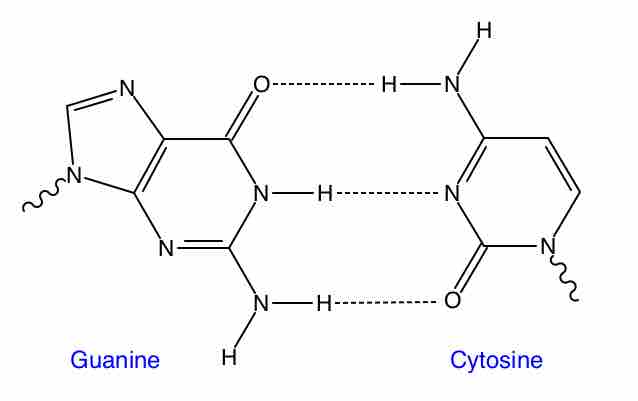
Hydrogen bonding between Guanine and Cytosine
In the instance of guanine and cytosine, there are three instances in which hydrogen atoms are attracted to nearby nitrogen and oxygen atoms (denoted by dashed lines).
When DNA replicates, as it does in cellular reproduction, it is first "unzipped" by any of a number of enzymes known as DNA Helicases. These enzymes break the electrostatic hydrogen bonds between the two strands.