A black body in thermal equilibrium (i.e. at a constant temperature) emits electromagnetic radiation called black body radiation. Black body radiation has a characteristic, continuous frequency spectrum that depends only on the body's temperature. Max Planck, in 1901, accurately described the radiation by assuming that electromagnetic radiation was emitted in discrete packets (or quanta). Planck's quantum hypothesis is a pioneering work, heralding advent of a new era of modern physics and quantum theory.
Explaining the properties of black-body radiation was a major challenge in theoretical physics during the late nineteenth century. Predictions based on classical theories failed to explain black body spectra observed experimentally, especially at shorter wavelength. The puzzle was solved in 1901 by Max Planck in the formalism now known as Planck's law of black-body radiation. Contrary to the common belief that electromagnetic radiation can take continuous values of energy, Planck introduced a radical concept that electromagnetic radiation was emitted in discrete packets (or quanta) of energy. Although Planck's derivation is beyond the scope of this section (it will be covered in Quantum Mechanics), Planck's law may be written:
where
Black body radiation spectrum
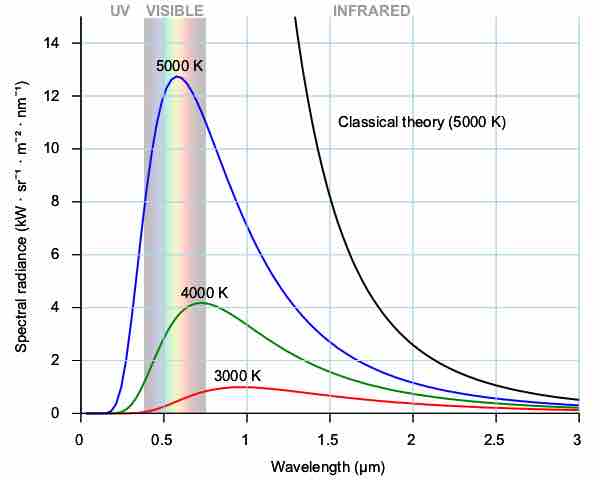
Typical spectrum from a black body at different temperatures (shown in blue, green and red curves). As the temperature decreases, the peak of the black-body radiation curve moves to lower intensities and longer wavelengths. Black line is a prediction of a classical theory for an object at 5,000K, showing catastropic discrepancy at shorter wavelengh.
Note that the spectral radiance depends on two variables, wavelength and temperature. The radiation has a specific spectrum and intensity that depends only on the temperature of the body. Despite its simplicity, Planck's law describes radiation properties of objects (e.g. our body, planets, stars) reasonably well.