The nitrogen cycle describes the conversion of nitrogen between different chemical forms. The majority of the earth's atmosphere (about 78%) is composed of atmospheric nitrogen, but it is not in a form that is usable to living things. Complex species interactions allow organisms to convert nitrogen to usable forms and exchange it between themselves. Nitrogen is essential for the formation of amino acids and nucleotides. It is essential for all living things.
Fixation: In order for organisms to use atmospheric nitrogen (N2), it must be "fixed" or converted into ammonia (NH3). This can happen occasionally through a lightning strike, but the bulk of nitrogen fixation is done by free living or symbiotic bacteria. These bacteria have the nitrogenase enzyme that combines gaseous nitrogen with hydrogen to produce ammonia. It is then further converted by the bacteria to make their own organic compounds. Some nitrogen fixing bacteria live in the root nodules of legumes where they produce ammonia in exchange for sugars. Today, about 30% of the total fixed nitrogen is manufactured in chemical plants for fertilizer .
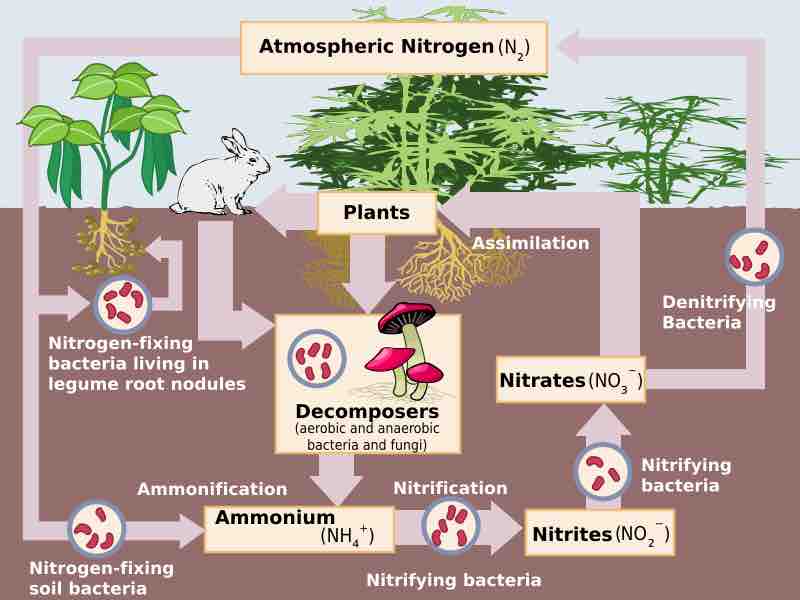
The role of soil bacteria in the Nitrogen cycle
Nitrogen transitions between various biologically useful forms.
Nitrificaton: Nitrification is the conversion of ammonia (NH3) to nitrate (NO3-). It is usually performed by soil living bacteria, such as nitrobacter. This is important because plants can assimilate nitrate into their tissues, and they rely on bacteria to convert it from ammonia to a usable form. Nitrification is performed mainly by the genus of bacteria, Nitrobacter.
Ammonification/Mineralization: In ammonification, bacteria or fungi convert the organic nitrogen from dead organisms back into ammonium (NH4+). Nitrification can also work on ammonium. It can either be cycled back into a plant usable form through nitrification or returned to the atmosphere through de-nitrification.
De-Nitrification: Nitrogen in its nitrate form (NO3-) is converted back into atmospheric nitrogen gas (N2) by bacterial species such as Pseudomonas and Clostridium, usually in anaerobic conditions. These bacteria use nitrate as an electron acceptor instead of oxygen during respiration.