Structural determination utilizing isotopes is often performed using two analytical techniques: nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry (MS).
Nuclear Magnetic Resonance Spectroscopy
NMR analysis is isotope-dependent, and it often relies on trace isotopes of a molecule for detection. For example, the most abundant isotope of carbon, C-12, is invisible to NMR, whereas the minor isotope C-13 is NMR active, but only comprises 1.1 percent of a given sample of carbon. By replacing C-12 in a molecule with C-13, NMR analysis of that position is greatly enhanced. Similarly, H-1 is an NMR active nucleus, whereas H-2 is NMR invisible, so it is possible to determine where a specific hydrogen atom is by replacing it with H-2 and watching for the disappearance of the corresponding signal.
Mass Spectrometry
Mass spectrometry is a technique for determining the molecular weight of an ionized molecule and fragments of the molecule that appear when the molecule is ionized. The addition of an isotope will change the observed mass of the parent ion—the molecule that is ionized and does not fragment. Adding an isotope will also change the observed mass of any fragment which contains the isotope. If a fragment does not contain the isotope, then its mass will not be changed. This can reveal information about the position of an isotope in a molecule.
Isotopic Labeling
Isotopic labeling is used to track the passage of an isotope through a reaction, metabolic pathway, or cell. This leads to information about not only the identity of the reaction product, but also provides information about the mechanism of the reaction or biochemical pathway. This method is commonly used in organic chemistry and biochemistry. The reactant is 'labeled' by replacing specific atoms with their isotope. The reaction is allowed to occur and the position of the isotopes in the products is measured; this shows the sequence that the isotopic atom followed during the reaction.
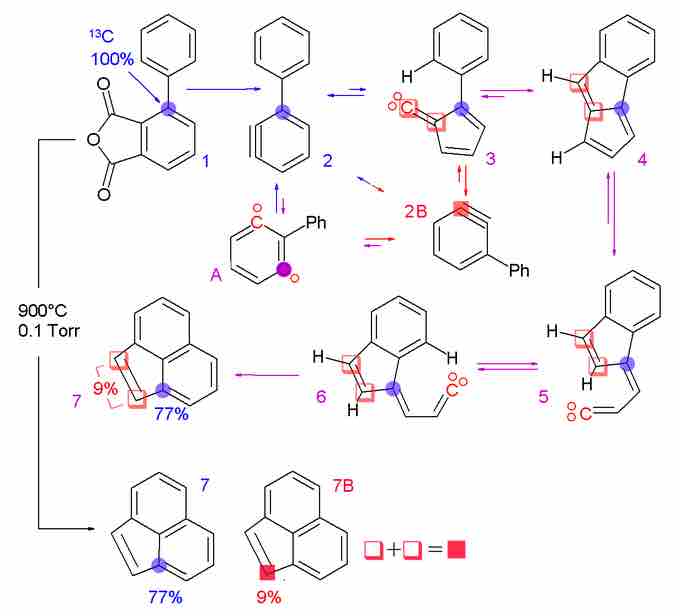
Isotopic labeling
A carbon-13 labeled precursor was used to track the position of an atom through a reaction.
In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes: mass, vibrational mode, or radioactive decay. Mass spectrometry and nuclear magnetic resonance detect the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.
An isotopic tracer is used in chemistry and biochemistry to help understand chemical reactions and interactions. In this technique, one or more atoms of a molecule are substituted for a different isotope of the same chemical element. Since the labeled atom—the isotope—has the same number of protons and electrons, it will behave in almost exactly the same way as its unlabeled counterpart. It will not interfere with the reaction being studied. The difference in the number of neutrons, however, means that it can be detected separately from the other atoms of the same element.
Nuclear magnetic resonance and mass spectrometry are used to investigate the mechanisms of chemical reactions. NMR and MS detect isotopic differences; detecting these differences allows information about the position atoms in a product's structure to be determined. With information about the position of isotopic atoms in products, the reaction pathway can also be determined.