Nuclear Fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse," to form a single heavier nucleus. During this process, matter is not conserved because some of the mass of the fusing nuclei is converted to energy, which is released. Fusion is the process that powers active stars, releasing large quantities of energy.
The origin of the energy released in fusion of light elements is due to an interplay of two opposing forces: the nuclear force that draws together protons and neutrons, and the Coulomb force that causes protons to repel each other. The protons are positively charged and repel each other, but they nonetheless stick together, demonstrating the existence of another force referred to as nuclear attraction. This force, called the strong nuclear force, overcomes electric repulsion in a very close range.
Fusion and Strong Nuclear Force
The effect of nuclear force is not observed outside the nucleus, hence the force has a strong dependence on distance; it a short-range force. The same force also pulls the nucleons, or neutrons and protons, together. The nuclear force is stronger than the Coulomb force for atomic nuclei smaller than iron, so building up these nuclei from lighter nuclei by fusion releases the extra energy from the net attraction of these particles. For larger nuclei, no energy is released, since the nuclear force is short-range and cannot continue to act across an even larger atomic nuclei. Therefore, energy is no longer released when such nuclei are made by fusion; instead, energy is absorbed.
Examples of Fusion
Fusion reactions of light elements power the stars and produce virtually all elements in a process called nucleosynthesis. The fusion of lighter elements in stars releases energy, as well as the mass that always accompanies it. For example, in the fusion of two hydrogen nuclei to form helium, seven-tenths of one percent of the mass is carried away from the system in the form of kinetic energy or other forms of energy, like electromagnetic radiation.
Requirements
A substantial energy barrier of electrostatic forces must be overcome before fusion can occur. At large distances, two nuclei repel one another because of the repulsive electrostatic force between their positively charged protons. If two nuclei can be brought close enough together, however, the electrostatic repulsion can be overcome by the attractive nuclear force, which is stronger at close distances.
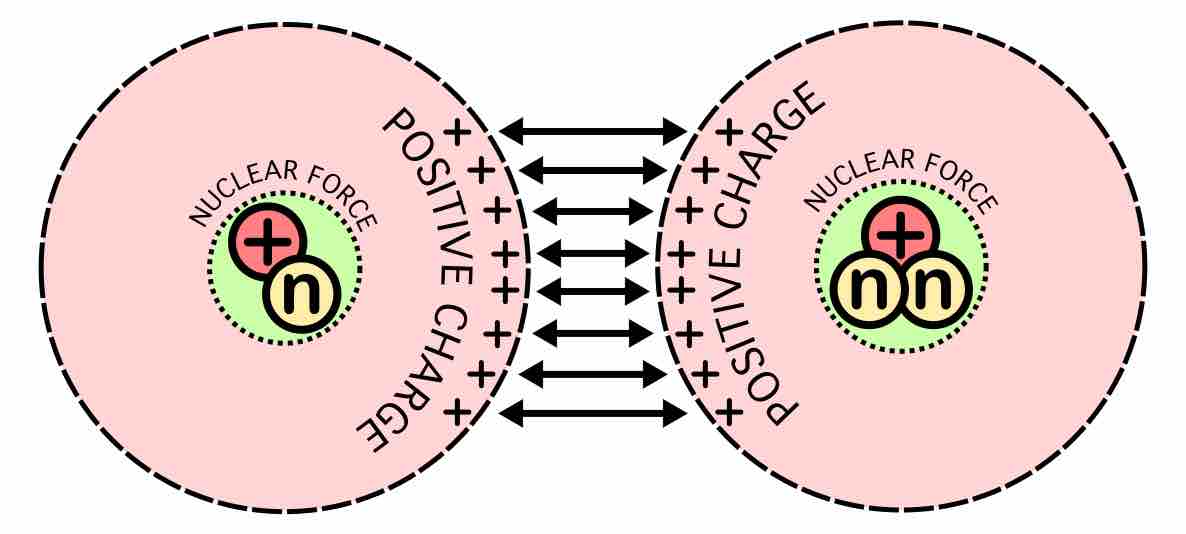
Nuclear fusion forces diagram
At nucleus radii distances, the attractive nuclear force is stronger than the repulsive electrostatic force. Therefore, the main technical difficulty for fusion is getting the nuclei close enough to fuse.
When a nucleon is added to a nucleus, the nuclear force attracts it to other nucleons, but primarily to its immediate neighbors due to the short range of the force. The nucleons in the interior of a nucleus have more neighboring nucleons than do those on the surface. The binding energy per nucleon generally increases with the size of the nucleus but approaches a limiting value corresponding to that of a nucleus with a diameter of about four nucleons.
The electrostatic force, on the other hand, is dependent upon the inverse-square of the distance between two like-charged particles, so a proton added to a nucleus will feel an electrostatic repulsion from all the other protons in the nucleus. The electrostatic energy per nucleon increases without limit as nuclei get larger due to the electrostatic force.