Concept
Version 13
Created by Boundless
Liquid to Gas Phase Transition
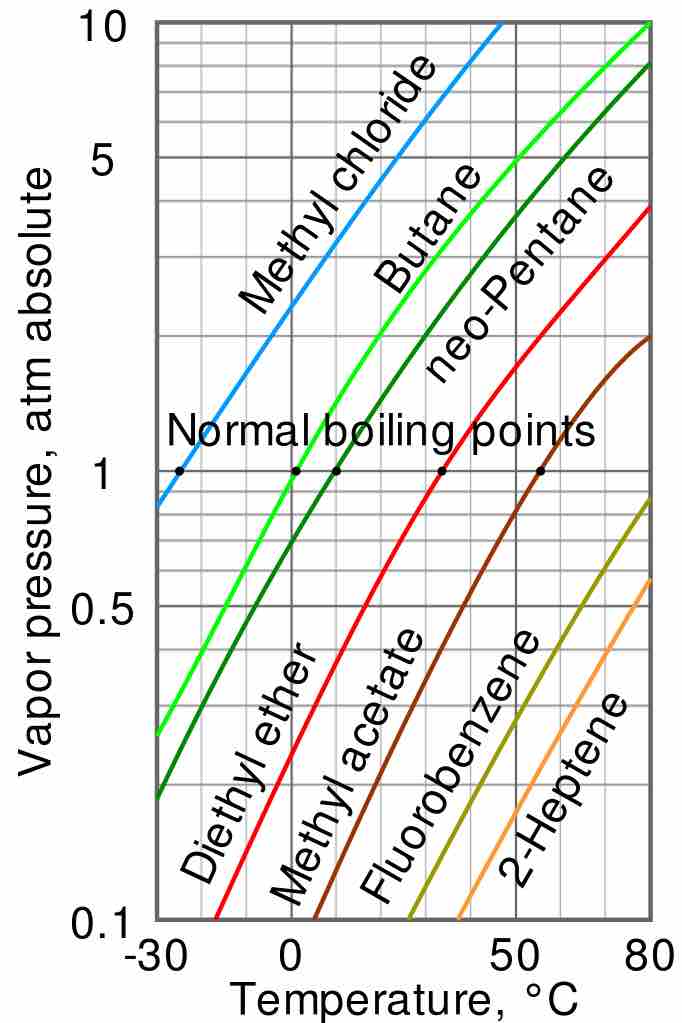
Vapor pressure and temperature
The variation of vapor pressure with temperature is not linear. The intercepts of each curve with the horizontal line at 1 atm (i.e. 760 torr) indicate the normal boiling point of each liquid, ranging from -25 °C for methyl chloride to over 80 °C for fluorobenzene and 2-heptene.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"682px-Vapor_pressure_chart.svg.png."
https://commons.wikimedia.org/wiki/File:Vapor_pressure_chart.svg
Wikimedia
CC BY-SA 3.0.