Section 2
Intermolecular Forces
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts
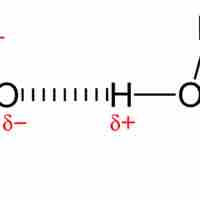
Dipole-Dipole Force
Dipole-dipole interactions are intermolecular attractions that result from two permanent dipoles interacting.
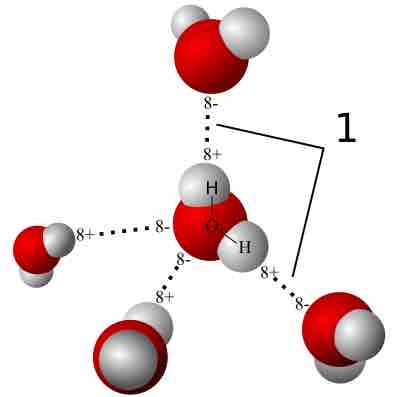
Hydrogen Bonding
A hydrogen bond is a strong intermolecular force created by the relative positivity of hydrogen atoms.

Ion-Dipole Force
The ion-dipole force is an intermolecular attraction between an ion and a polar molecule.

Dispersion Force
Dispersion forces are weak intermolecular forces caused by temporary dipoles.