When space shuttles return from space and being to re-enter the Earth's atmosphere, a glow, especially around the tail end of the shuttle, can often be observed. This phenomenon has to do with the composition of the atmosphere at these altitudes and the lack of shielding from the sun.
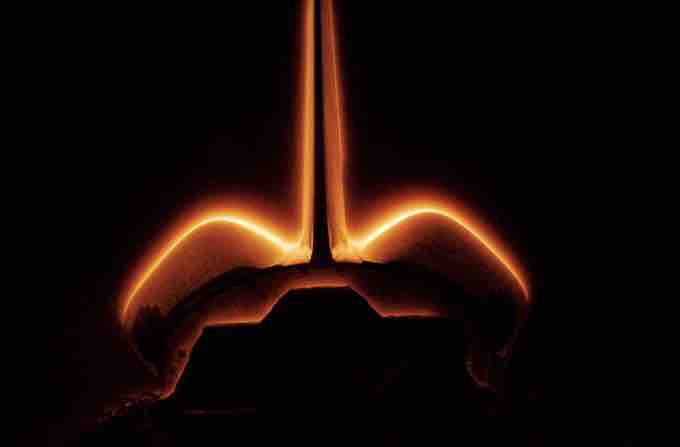
Shuttle glow
When atomic oxygen from the high atmosphere combines with nitric oxide on the surface of the space shuttle, the resulting excited nitrogen dioxide returns to the ground state emitting an apparent glow.
Where Does the Glow Come From?
You may have heard of the ozone layer, which protects the Earth from ultraviolet radiation. This protection is not present higher up in the atmosphere, however, where the high-energy light from the sun interacts with molecular oxygen, breaking it apart into atomic oxygen. This highly-reactive species interacts with almost any molecule with which it comes in contact.
The lining of the space shuttle is no exception; specifically, the glow on the space shuttle forms when atomic oxygen reacts with the nitric oxide (NO) on the exterior of the shuttle. The origin of the NO is unclear, although it may be collected from the atmosphere or be a byproduct of the fuel consumption from the shuttle thrusters. This interaction produces NO2 according to the equation:
where NO2* represents the excited state of electrons in NO2. It is the relaxation of these electrons from the excited state back to the ground state that produces the glow that is visible around the space shuttle (see the concept about the emission spectra for more information).