The Effect of a Catalyst on Equilibrium
Reactions can be sped up by the addition of a catalyst, including reversible reactions involving a final equilibrium state. Recall that for a reversible reaction, the equilibrium state is one in which the forward and reverse reaction rates are equal. In the presence of a catalyst, both the forward and reverse reaction rates will speed up equally, thereby allowing the system to reach equilibrium faster. However, it is very important to keep in mind that the addition of a catalyst has no effect whatsoever on the final equilibrium position of the reaction. It simply gets it there faster.
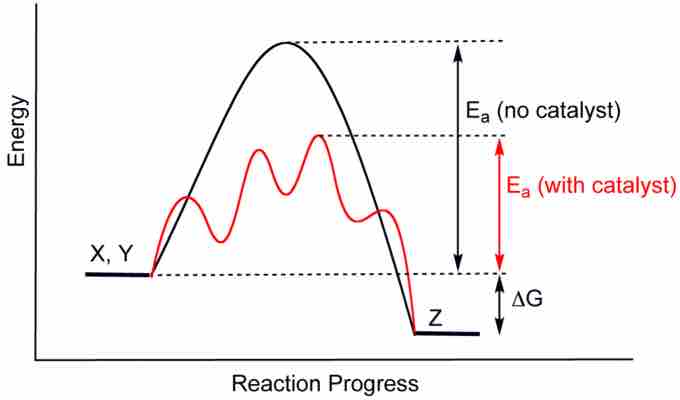
Recall that catalysts are compounds that accelerate the progress of a reaction without being consumed. Common examples of catalysts include acid catalysts and enzymes. Catalysts allow reactions to proceed faster through a lower-energy transition state. By lowering the energy of the transition state, which is the rate-limiting step, catalysts reduce the required energy of activation to allow a reaction to proceed and, in the case of a reversible reaction, reach equilibrium more rapidly.
Catalysis
A catalyst speeds up a reaction by lowering the activation energy required for the reaction to proceed.
To reiterate, catalysts do not affect the equilibrium state of a reaction. In the presence of a catalyst, the same amounts of reactants and products will be present at equilibrium as there would be in the uncatalyzed reaction. To state this in chemical terms, catalysts affect the kinetics, but not the thermodynamics, of a reaction. If the addition of catalysts could possibly alter the equilibrium state of the reaction, this would violate the second rule of thermodynamics; we would be getting "something for nothing," which is physically impossible.