Acid-Base Titrations
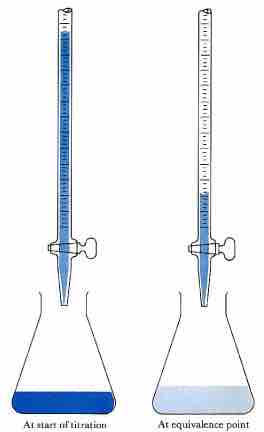
Acid-base titration
The solution in the flask contains an unknown number of equivalents of base (or acid). The burette is calibrated to show volume to the nearest 0.001 cm3. It is filled with a solution of strong acid (or base) of known concentration. Small increments are added from the burette until, at the end point, one drop changes the indicator color permanently. (An indication of the approaching equivalence point is the appearance, and disappearance after stirring, of the color that the indicator assumes beyond neutralization.) At the equivalence point, the total amount of acid (or base) is recorded from the burette readings. The number of equivalents of acid and base must be equal at the equivalence point.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: